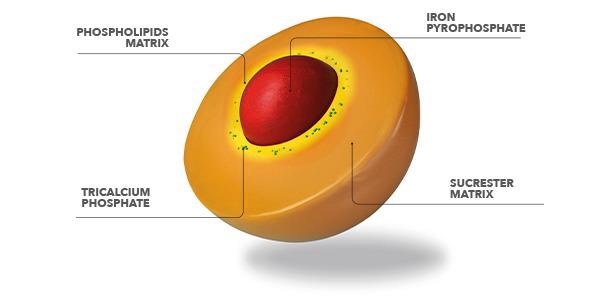
Sucrosomial® iron is an progressive oral iron expertise, during which ferric pyrophosphate (trivalent iron) is transported inside a matrix of phospholipids and sucrose esters of fatty acids. This construction, known as Sucrosome®, permits excessive absorption of iron and optimum gastrointestinal tolerability.
Recent Articles
Iron deficiency (ID)
Iron is an important component for the physique. It promotes the formation of hemoglobin and myoglobin, helps the immune system and regular cognitive operate in addition to supporting progress in kids and adolescents.
The causes that may result in the rise within the want for iron are completely different, however, in all circumstances, it’s mandatory to revive the physique’s stability of this nutrient so as to facilitate sure regular physiological capabilities of our our bodies.
Iron deficiency (ID) is a situation characterised by a low degree of this mineral within the physique, that, if not recovered, results in an anemic scientific situation. Iron deficiency anemia (IDA) happens when the physique will not be in a position to fulfill a proper hemoglobin manufacturing, which is pivotal to make sure the oxygenation of tissues and cells of the physique.
The principle causes of ID are elevated demand, lowered absorption, and elevated lack of iron.
Within the image under is proven the presence of ID on particular scientific situations:
Supply: Susana Gómez-Ramírez et al., Sucrosomial® Iron: A New Technology Iron for Bettering Oral Supplementation.
World Health Organization (WHO) has defined the values of hemoglobin (Hb) as normal range at 13-18 g/dL for men and 12-16 g/dL for women; below these values, iron deficiency anemia is diagnosed. Other important markers to be considered for ID anemia are: ferritin, a storage protein able to contain up to 4500 iron atoms, transferrin saturation (%TSAT), as this glycoprotein is able to transport iron to the tissues and body districts that use this mineral, and sideremia, which indicates the amount of iron bound to transferrin in the bloodstream.
Iron Deficiency itself, instead, doesn’t always show hematological alterations; in fact, the hemoglobin level can be within the normal range, but the ferritin and the saturation of transferrin can be below. If the deficiency is not timely adjusted, it could develop in IDA.
The common symptoms related with ID and IDA, are:
- Paleness;
- Weak point;
- Dizziness;
- Irritability;
- Problem concentrating.
Commonly, ID is treated with oral iron salts (e.g. iron sulfate); however, conventional oral iron supplements are poorly absorbed by the body and lead to gastrointestinal side effects, with consequently reduced compliance and frequent drop outs by the patients.
Sucrosomial® Iron (Sideral ®)
This new patented technology represents an innovative oral iron-containing carrier, in which ferric pyrophosphate is transported within a phospholipid plus sucrester matrix.
The result is a high gastrointestinal tolerability and easier iron absorption at the intestinal level. In the recent years many scientific evidences (each preclinical and scientific trials) have been printed on Worldwide Journals confirming Sucrosomial® expertise effectiveness.
Gastro-resistance and Absorption
The gastro-resistance is due primarily to the sucrester matrix, as demonstrated by in vitro research, that protects the iron from the acid gastric fluid.
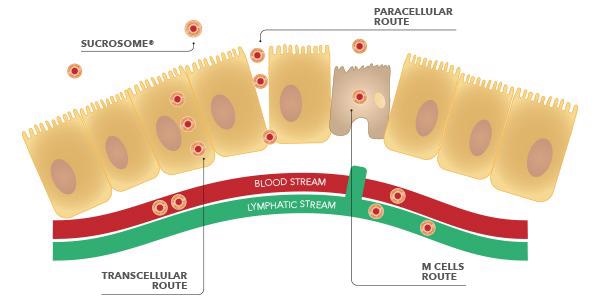
This enables the Sucrosome® to completely attain the intestinal mucosa, the place it’s absorbed by enterocytes by way of 2 routes: para-cellular and trans-cellular.
Moreover, within the intestinal tract, additionally the Membranous cells (M cells) of the Peyer’s patches are concerned for Sucrosomial® iron absorption. Research have demonstrated, in actual fact, that these cells are ready to participate to the absorption of the Sucrosome® and to switch it to the lymphatic system.
Distribution
Scientific evidences on Sucrosomial® iron demonstrate that this newer technology allows the absorption of iron by the intestinal tract and its distribution to the target tissues.
Considering its structure, similar to chylomicrons (gut lypoproteins able to transport lipophilic substances), it can be assumed that Sucrosomial® iron reaches the liver, where the epatocytes are able to metabolize the phospholipid matrix, thanks to lysosomial enzymes, and to make available the ferric pyrophosphate for transferrin bound, and consequent tissues distribution, in particular to the bone marrow to form new red blood cells.
Iron Deficiency in Clinical Practice
Several studies in scientific literature confirm the efficacy of Sucrosomial® iron supplementation in lots of scientific settings, the place the deficiency of iron is frequent, for instance: ginecology, oncology, nephrology, cardiology and particularly in gastroenterology.
In gastroenterology, ID and IDA are probably the most frequent systemic issues in Inflammatory Bowel Illness (IBD), celiac illness and weight problems. All these pathologies result in continual irritation, that limits absorption and use of iron by the physique. In sufferers with practical ID, the supplementation of standard oral iron normally isn’t in a position to resolve this drawback, as a result of proinflammatory cytokines are produced by the organism after irritation. These cytokines stimulate the manufacturing and improve of hepcidin ranges, a peptide hormone produced by the liver, which works by lowering serum iron focus. Many research in gastroenterology show that the supplementation with Sucrosomial® iron permits to revive regular hematological ranges, and consequently to grant the traditional operate of purple blood cells and hemoglobin.
Conclusion
The innovation of Sucrosomial® technology is characterized by an excellent tolerability and it allows the iron intake anytime in the day (with meal or far from meals), for long periods of time, and prevents any discomfort commonly associated with iron intake, such as metallic and unpleasant aftertaste, irritation of the gastric mucosa, nausea or constipation. Overcoming the limits related to the conventional supplementation of iron, Sucrosomial® iron promotes the intake of this important nutrient in all situations of deficiency or increased needs for iron.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
References:
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.Ok.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A scientific evaluation of worldwide anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Illness and Damage Incidence and Prevalence Collaborators. International, regional, and nationwide incidence, prevalence, and years lived with incapacity for 328 illnesses and accidents for 195 international locations, 1990–2016: A scientific evaluation for the International Burden of Illness Research 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Remacha-Sevilla, Á.F.; Muñoz-Gómez, M. Anaemia within the aged. Med. Clin. 2017, 149, 496–503. [Google Scholar] [CrossRef]
- Muñoz, M.; García-Erce, J.A.; Remacha, A.F. Problems of iron metabolism. Half II: Iron deficiency and iron overload. J. Clin. Pathol. 2011, 64, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Peña-Rosas, J.P.; Robinson, S.; Milman, N.; Holzgreve, W.; Breymann, C.; Goffinet, F.; Nizard, J.; Christory, F.; Samama, C.M.; et al. Affected person blood administration in obstetrics: Administration of anaemia and haematinic deficiencies in being pregnant and within the post-partum interval: NATA consensus assertion. Transfus. Med. 2018, 28, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascon, P.; Glaspy, J.; Hofmann, A.; Hyperlink, H.; Littlewood, T.; Ludwig, H.; et al. Administration of anaemia and iron deficiency in sufferers with most cancers: ESMO Medical Follow Tips. Ann. Oncol. 2018. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bircher, A.J.; Eckardt, Ok.U.; Obrador, G.T.; Pollock, C.A.; Stenvinkel, P.; Swinkels, D.W.; Wanner, C.; Weiss, G.; Chertow, G.M.; et al. Iron administration in continual kidney illness: Conclusions from a “Kidney Illness: Bettering International Outcomes” (KDIGO) Controversies Convention. Kidney Int. 2016, 89, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Comin-Colet, J.; Leal-Noval, S.; Ozawa, S.; Takere, J.; Henry, D.; Javidroozi, M.; Hohmuth, B.; Bisbe, E.; Gross, I.; et al. Administration of anemia in sufferers with congestive coronary heart failure. Am. J. Hematol. 2017, 92, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegard, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, Ok.; Koutroubakis, I.; et al. European consensus on the prognosis and administration of iron deficiency and anaemia in inflammatory bowel illnesses. J. Crohns Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Botella-Romero, F.; Gómez-Ramírez, S.; Campos, A.; Garcia-Erce, J.A. Iron deficiency and anaemia in bariatric surgical sufferers: Causes, prognosis and correct administration. Nutr. Hosp. 2009, 24, 640–654. [Google Scholar] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency throughout continual inflammatory situations: Worldwide professional opinion on definition, prognosis, and administration. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muñoz, M.; Gómez-Ramírez, S.; Campos, A.; Ruiz, J.; Liumbruno, G.M. Pre-operative anaemia: Prevalence, penalties and approaches to administration. Blood Transfus. 2015, 13, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cladellas, M.; Nuñez-Matas, M.J.; Garcia-Erce, J.A. Pre-operative haemoglobin ranges and iron standing in a big multicentre cohort of sufferers present process main elective surgical procedure. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Adamson, J.W. How we diagnose and deal with iron deficiency anemia. Am. J. Hematol. 2016, 91, 31–38. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Iolascon, A.; Taher, A.; Cappellini, M.D. Medical administration of iron deficiency anemia in adults: Systemic evaluate on advances in prognosis and therapy. Eur. J. Intern. Med. 2017, 42, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Gómez-Ramírez, S.; Besser, M.; Pavia, J.; Gomollon, F.; Liumbruno, G.M.; Bhandari, S.; Cladellas, M.; Shander, A.; Auerbach, M. Present misconceptions in prognosis and administration of iron deficiency. Blood Transfus. 2017, 15, 422–437. [Google Scholar] [CrossRef] [PubMed]
- World Well being Group (WHO). Haemoglobin Concentrations for the Analysis of Anaemia and Evaluation of Severity. WHO/NMH/NHD/MNM/11.1. Out there on-line: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 15 April 2018).
- Pratt, J.J.; Khan, Ok.S. Non-anaemic iron deficiency—A illness in search of recognition of prognosis: A scientific evaluate. Eur. J. Haematol. 2016, 96, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Cook dinner, J.D. Analysis and administration of iron-deficiency anaemia. Greatest Pract. Res. Clin. Haematol. 2005, 18, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Rao Baikady, R.; et al. Worldwide consensus assertion on the peri-operative administration of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Walters, G.O.; Miller, F.M.; Worwood, M. Serum ferritin focus and iron shops in regular topics. J. Clin. Pathol. 1973, 26, 770–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barni, S.; Gascon, P.; Petrelli, F.; Garcia-Erce, J.A.; Pedrazzoli, P.; Rosti, G.; Giordano, G.; Mafodda, A.; Munoz, M. Place paper on administration of iron deficiency in grownup most cancers sufferers. Professional Rev. Hematol. 2017, 10, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Gómez-Ramírez, S.; Bhandari, S. The protection of accessible therapy choices for iron-deficiency anemia. Professional Opin. Drug Saf. 2018, 17, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous sulfate supplementation causes vital gastrointestinal side-effects in adults: A scientific evaluate and meta-analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [PubMed]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Perez-Edo, L. Tolerability of various oral iron dietary supplements: A scientific evaluate. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron dietary supplements improve hepcidin and reduce iron absorption from each day or twice-daily doses in iron-depleted younger girls. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron dietary supplements given on consecutive versus alternate days and as single morning doses versus twice-daily break up dosing in iron-depleted girls: Two open-label, randomised managed trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving an excessive amount of iron? Low-dose iron remedy is efficient in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Company. New Suggestions to Handle Danger of Allergic Reactions with Intravenous Iron Containing Medicines. EMA/579491/2013. Out there on-line: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500151308.pdf (accessed on 18 July 2018).
- Leal-Noval, S.R.; Muñoz, M.; Asuero, M.; Contreras, E.; Garcia-Erce, J.A.; Llau, J.V.; Ethical, V.; Paramo, J.A.; Quintana, M. Spanish Professional Panel on Options to Allogeneic Blood T Spanish Consensus Assertion on options to allogeneic blood transfusion: The 2013 replace of the “Seville Doc”. Blood Transfus. 2013, 11, 585–610. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Marano, G.; Mengoli, C.; Pupella, S.; Vaglio, S.; Munoz, M.; Liumbruno, G.M. Purple blood cell transfusion coverage: A essential literature evaluate. Blood Transfus. 2017, 15, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.Ok.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Medical Follow Tips From the AABB: Purple Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Vaglio, S.; Gentili, S.; Marano, G.; Pupella, S.; Rafanelli, D.; Biancofiore, G.; Antonioli, P.; Velati, C.; Liumbruno, G.M. The Italian Regulatory Tips for the implementation of Affected person Blood Administration. Blood Transfus. 2017, 15, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Fashionable iron substitute remedy: Medical and pathophysiological insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Kis, L.; Szuts, A.; Otomo, N.; Szabo-Rvevz Deli, M.A. The Potential of sucrose esters for use as oral absorption enhacers. Sci. Pharm. 2010, 78, 716. [Google Scholar] [CrossRef]
- Kis, L.; Hellinger, E.; Pilbat, A.M.; Kittel, A.; Torok, Z.; Furedi, A.; Szakacs, G.; Veszelka, S.; Sipos, P.; Ozsvari, B.; et al. Sucrose esters improve drug penetration, however don’t inhibit p-glycoprotein in Caco-2 intestinal epithelial cells. J. Pharm. Sci. 2014, 103, 3107–3119. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, N.; Satsu, H.; Shimizu, M. Enhanced daunomycin accumulation in human intestinal Caco-2 cells from non-ionic meals emulsifiers unrelated to the p-glycoprotein inhibitory mechanism. Biosci. Biotechnol. Biochem. 2006, 70, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Quintanar-Guerrero, D.; Ganem-Quintanar, A.; Allemann, E.; Fessi, H.; Doelker, E. Affect of the stabilizer coating layer on the purification and freeze-drying of poly(d,l-lactic acid) nanoparticles ready by an emulsion-diffusion method. J. Microencapsul. 1998, 15, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Fogli, S.; Beconcini, D.; Carpi, S.; Tarantino, G.; Zambito, Y. Sucrosomial® iron absorption studied by in vitro and ex-vivo fashions. Eur. J. Pharm. Sci. 2018, 111, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Brilli, E.; Romano, A.; Fabiano, A.; Zambito, Y.; Di Raimondo, F.; Tarantino, G. Sucrosomial® expertise is ready to promote ferric iron absorption: Pre-clinical and scientific evidences. Blood 2016, 128, 3618. [Google Scholar]
- Fabiano, A.; Brilli, E.; Mattii, L.; Testai, L.; Moscato, S.; Citi, V.; Tarantino, G.; Zambito, Y. Ex vivo and in vivo examine of Sucrosomial® iron intestinal absorption and bioavailability. Int. J. Mol. Sci. 2018, 19, 2722. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Plapied, L.; Plaideau, C.; Legendre, D.; des Rieux, A.; Pourcelle, V.; Freichels, H.; Jerome, C.; Marchand, J.; Preat, V.; et al. In vitro identification of focusing on ligands of human M cells by phage show. Int. J. Pharm. 2010, 394, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Vital immunosurveillance posts within the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tarantino, G.; Brilli, E.; Zambito, Y.; Giordano, G.; Equitani, F. Sucrosomial® iron: A brand new extremely bioavailable oral iron complement. Blood 2015, 126, 4561–4562. [Google Scholar]
- Starzynski, R.; Szudzik, M.; Staron, R.; Jonczy, A.; Smuda, E.; Pieszka, M.; Kamyczek, M.; Lipinski, P. Comparability of the therapeutical potential of oral Sucrosomial® iron and parenteral iron dextran supplementations in neonatal iron deficiency anemia in pigs. Am. J. Hematol. 2017, 92, E286. [Google Scholar]
- Asperti, A.; Gryzik, M.; Brilli, E.; Castagna, A.; Corbella, M.; Gottardo, R.; Girelli, D.; Tarantino, G.; Arosio, P.; Poli, M. Sucrosomial® iron supplementation in mice: Results on blood parameters, hepcidin, and irritation. Vitamins 2018, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Ferretti, F.; Branchi, F.; Tomba, C.; Lombardo, V.; Scricciolo, A.; Doneda, L.; Roncoroni, L. Sucrosomial® iron supplementation in anemic sufferers with celiac illness not tolerating oral ferrous sulfate: A potential examine. Vitamins 2018, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Simo-Servat, O.; Balibrea, J.M.; Vilallonga, R.; Hernandez, C.; Simo, R.; Mesa, J. Response to oral Sucrosomial® iron supplementation in sufferers present process bariatric surgical procedure. The BARI-FER examine. Endocrinol. Diabetes Nutr. 2018, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Impact of oral liposomal iron versus intravenous iron for therapy of iron deficiency anaemia in CKD sufferers: A randomized trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Mafodda, A.; Giuffrida, D.; Prestifilippo, A.; Azzarello, D.; Giannicola, R.; Mare, M.; Maisano, R. Oral Sucrosomial® iron versus intravenous iron in anemic most cancers sufferers with out iron deficiency receiving darbepoetin alfa: A pilot examine. Assist. Care Most cancers 2017, 25, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Capra, A.P.; Ferro, E.; Cannavò, L.; La Rosa, M.A.; Zirilli, G. A baby with extreme iron-deficiency anemia and a posh TMPRSS6 genotype. Hematology 2017, 22, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Brilli, E.; Barnadas, R.; Camacho, M.; Giordano, G.; Tarantino, G. Sucrosomial® Iron Absorption Includes M Cells Interplay. In Proceedings of the European Iron Membership Annual Assembly, Zürich, Switzerland, 8–11 February 2018; p. 51. [Google Scholar]
- Rishi, G.; Subramaniam, V.N. The liver in regulation of iron homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G157–G165. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of irritation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toblli, J.E.; Cao, G.; Olivieri, L.; Angerosa, M. Comparative examine of gastrointestinal tract and liver toxicity of ferrous sulfate, iron amino chelate and iron polymaltose advanced in regular rats. Pharmacology 2008, 82, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nakanishi, H. Phosphatidylserine-containing liposomes: Potential pharmacological interventions towards inflammatory and immune illnesses by way of the manufacturing of prostaglandin E(2) after uptake by myeloid derived phagocytes. Arch. Immunol. Ther. Exp. 2011, 59, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Daru, J.; Zamora, J.; Fernandez-Felix, B.M.; Vogel, J.; Oladapo, O.T.; Morisaki, N.; Tuncalp, O.; Torloni, M.R.; Mittal, S.; Jayaratne, Ok.; et al. Danger of maternal mortality in girls with extreme anaemia throughout being pregnant and postpartum: A multilevel evaluation. Lancet Glob. Well being 2018, 6, e548–e554. [Google Scholar] [CrossRef]
- Fonseca, C.; Araujo, M.; Moniz, P.; Marques, F.; Araujo, I.; Costa, L.; Rodrigues, J.; Frade, L.; Botella, A.; Jesus, S.; et al. Prevalence and prognostic affect of anemia and iron deficiency in sufferers hospitalized in an inside drugs ward: The PRO-IRON examine. Eur. J. Haematol. 2017, 99, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Berti, C.; Mando, C.; Martinelli, A.; Mazzali, C.; Cetin, I. Results of various regimens of iron prophylaxis on maternal iron standing and being pregnant final result: A randomized management trial. J. Matern. Fetal Neonatal Med. 2017, 30, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Berardi, S.; Foltran, L.; Pascoli, I.; Pepe, A.; Salmeri, M.G.; Busato, E. Efficacy of oral Sucrosomial® iron in puerperium anemia. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), 40. [Google Scholar] [CrossRef]
- Barni, S.; Lonati, V.; Ghilardi, M.; Borgonovo, Ok.F.; Cabiddu, M.; Astori, A.; Tarantino, G.; Petrelli, F. Upfront use of Sucrosomial® iron prevents transfusions in most cancers sufferers on chemotherapy. Assist. Care Most cancers 2017, 25 (Suppl. S2), S144–S145. [Google Scholar]
- Locatelli, F.; Mazzaferro, S.; Yee, J. Iron Remedy Challenges for the Remedy of Nondialysis CKD Sufferers. Clin. J. Am. Soc. Nephrol. 2016, 11, 1269–1280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shepshelovich, D.; Rozen-Zvi, B.; Avni, T.; Gafter, U.; Gafter-Gvili, A. Intravenous versus oral iron supplementation for the therapy of anemia in CKD: An up to date systematic evaluate and meta-analysis. Am. J. Kidney Dis. 2016, 68, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kusek, J.W.; Pappas, M.Ok. A randomized trial of intravenous and oral iron in continual kidney illness. Kidney Int. 2015, 88, 905–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, Ok.U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G.; Roger, S.D.; Investigators F-CS. FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in sufferers with continual kidney illness and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014, 29, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, Ok.U.; Gaillard, C.; Wyck, D.V.; Meier, Y.; Larroque, S.; Perrin, A.; Roger, S.D. Erythropoietic response to oral iron in sufferers with nondialysis-dependent continual kidney illness within the FIND-CKD trial. Clin. Nephrol. 2017, 88, 301–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalantar-Zadeh, Ok.; Regidor, D.L.; McAllister, C.J.; Michael, B.; Warnock, D.G. Time-dependent associations between iron and mortality in hemodialysis sufferers. J. Am. Soc. Nephrol. 2005, 16, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Darba, J.; Ascanio, M. Finances Influence Evaluation of Oral Fisiogen Ferro Forte((R)) versus Intravenous Iron for the Administration of Iron Deficiency in Continual Kidney Illness in Spain. Clin. Drug Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Markopoulos, Ok.; Albertini, R.; Di Sabatino, A.; Biagi, F.; Ciccocioppo, R.; Arbustini, E.; Corazza, G.R. Anemia of continual illness and faulty erythropoietin manufacturing in sufferers with celiac illness. Haematologica 2008, 93, 1785–1791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, T.; Clavel, T.; Smirnov, Ok.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron substitute remedy distinctly alters the intestine microbiota and metabolome in sufferers with IBD. Intestine 2017, 66, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calve, A.; Santos, M.M. Iron Dietary supplements Modulate Colon Microbiota Composition and Potentiate the Protecting Results of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm. Bowel Dis. 2017, 23, 753–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stein, J.; Aksan, A.; Farrag, Ok.; Dignass, A.; Radeke, H.H. Administration of inflammatory bowel disease-related anemia and iron deficiency with particular reference to the function of intravenous iron in present follow. Professional Opin. Pharmacother. 2017, 18, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Bastida, G. Efficacy and tolerability of Sucrosomial iron supplementation in IBD sufferers with iron deficiency anemia and intolerance to iron oral salts. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), 6–8. [Google Scholar] [CrossRef]
- Stuklov, N.I.; Basiladze, I.G.; Pivnik, A.V.; Knyazev, O.V.; Parfenov, A.I. Traits and trendy therapy of iron deficiency syndromes in inflammatory bowel illnesses. Exp. Rev. Hematol. 2018, 10 (Suppl. S1). in press. [Google Scholar]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG scientific pointers: Analysis and administration of celiac illness. Am. J. Gastroenterol. 2013, 108, 656–676, quiz 677. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Camaschella, C. How I deal with unexplained refractory iron deficiency anemia. Blood 2014, 123, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Farinati, F.; Maddalo, G. Iron and/or B12 poor anemia in autoimmune gastritis. Excessive dose sucrosomial iron supplementation: Preliminary knowledge of a single middle expertise. Exp. Rev. Hematol. 2018, 10 (Suppl. S1). in press. [Google Scholar]
- Shipton, M.; Johal, N.; Dutta, N.; Ahmed, B.; Ammori, B.; Senapati, SP.; Akhtar, Ok.; Summers, L.; New, J.; Syed, A. Deficiencies of vitamin B12, folate and iron over 4 years of follow-up post-bariatric surgical procedure [abstract]. Br. J. Surg. 2018, 105 (Suppl. S4), 28. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Tips for the prognosis and therapy of acute and continual coronary heart failure: The Job Power for the prognosis and therapy of acute and continual coronary heart failure of the European Society of Cardiology (ESC)Developed with the particular contribution of the Coronary heart Failure Affiliation (HFA) of the ESC. Eur. Coronary heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M.; Tang, W.H.W.; LaRue, S.J.; Redfield, M.M.; Semigran, M.J.; et al. Impact of Oral Iron Repletion on Train Capability in Sufferers With Coronary heart Failure With Decreased Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Medical Trial. JAMA 2017, 317, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Karavidas, A.; Trogkanis, E.; Farmakis, D.; Papingiotis, G.; Matzaraki, V.; Perpinia, A.; Parissis, J. Oral sucrosomial iron improves high quality of life in coronary heart failure sufferers with iron deficiency: A preliminary proof-of-concept examine. Exp. Rev. Hematol. 2018, 10 (Suppl. S1). in press. [Google Scholar]
- Cabrera, P.; Gómez, S.; Herrero, V.; Martín, E.; Pavía, J.; Muñoz, M. Prevalence and penalties of anaemia amongst sufferers hospitalised on the inside drugs ward: A single centre audit. In Proceedings of the fifteenth European Congress of Inner Medication, Amsterdam, The Netherlands, 2–3 September 2016. [Google Scholar]
- Fonseca, C.; Marques, F.; Robalo Nunes, A.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of anaemia and iron deficiency in Portugal: The EMPIRE examine. Intern. Med. J. 2016, 46, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Mondello, P.; Tambaro, R.; Perrotta, N.; D’Amico, F.; D’Aveta, A.; Berardi, G.; Carabellese, B.; Patriarca, A.; Corbi, G.M.; et al. Biosimilar epoetin alpha is as efficient as originator epoetin-alpha plus liposomal iron (Sideral(R)), vitamin B12 and folates in sufferers with refractory anemia: A retrospective real-life strategy. Mol. Clin. Oncol. 2015, 3, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Santini, V. Medical use of erythropoietic stimulating brokers in myelodysplastic syndromes. Oncologist 2011, 16 (Suppl. S3), 35–42. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G. Discount of inflammatory markers with liposomal iron (Sideral®). Pre-clinical and scientific outcomes. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), S17. [Google Scholar] [CrossRef]
- Giordano, G. Oral high-dose Sucrosomial® Iron vs. intravenous iron in sideropenic anemia illiberal/refractory to iron sulfate. Multicentric randomized examine. Exp. Rev. Hematol. 2016, 9 (Suppl. S1), 15–17. [Google Scholar] [CrossRef]
- Giordano, G.; Parente, A.; Berardi, D.; Castaldi, D.; Cinotti, M.; Vedruccio, F.; Susca, V.; Petrella, L.; Berardi, G. Effectiveness of various oral iron formulations in iron deficiency anemia because of gastrointestinal bleeding: Multicentric randomized examine; European Hematology Affiliation: Stockholm, Sweden, 2018. [Google Scholar]
- Muñoz, M.; Franchini, M.; Liumbruno, G.M. The post-operative administration of anaemia: Extra efforts are wanted. Blood Transfus. 2018, 16, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Scardino, M.; Di Matteo, B.; Martorelli, F.; Tanzi, D.; Kon, E.; D’Amato, T. Improved affected person blood administration and price saving in hip substitute surgical procedure by way of the implementation of pre-operative Sucrosomial® iron supplementation: A top quality enchancment evaluation examine. Int. Orthop. 2018, 1–8, [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
Conflicts of Interest
S.G.-R. has nothing to declare; E.B. is an Alesco S.r.l. employee; G.T. is a Pharmanutra S.p.A. employee; M.M. has received industry-supplied honoraria for consultancies, lectures and/or travel support from Pharmacosmos, Vifor Pharma, Zambon, Pharmanutra, Sandoz and Celgene, and is member of the editorial board of the journals, “Revista Española de Anestesiología y Reanimación”, “Medicina Intensiva” and “Blood Transfusion”.



